Early benefit assessment of innovative pharmaceuticals according to AMNOG in Germany: empirical analysis of the decision-making process
Early benefit assessment of innovative pharmaceuticals according to AMNOG in Germany: empirical analysis of the decision-making process
In collaboration with: Universität Hamburg, Hamburg Center of Health Economics
Supported by: Deutsche Forschungsgemeinschaft

Project description
(FI 1950/1-1 und STA 1311/1-1)
Background
With the adoption of AMNOG in 2011, the framework conditions for the market access of the pharmaceutical industry to patented drugs changed significantly. Manufacturers must submit a dossier to the Federal Joint Committee within three months of market entry to prove the medical benefit and additional utility compared to standard therapy. Depending on the outcome of the benefit assessment, the manufacturer can then engage price negotiations with the GKV-Spitzenverband, the central interest group of the health insurance funds, or subjects further regulation. The project aims to identify influencing factors used to determine the additional benefit and the amount of the discount to be paid on the manufacturer's retail price using the existing benefit assessments since the beginning of 2011.
Objective
Therefore, based on the existing benefit assessments since the beginning of 2011, influencing factors for the determination of the additional benefit and the amount of the discount to be paid were identified, by applying regression analysis. The work programme described in the application was implemented accordingly. Using the AMNOG database, the course of the project engendered three separate research projects, which dealt with the following sub-aspects according to the work programme and with questions going beyond it:
1. Analysing the effects of the benefit assessment and / or the decision process on the price and the amount of the discount to be paid, respectively.
2. Early benefit assessment according to AMNOG - international comparison with decisions in England, Scotland and Australia
3. Impact of the evaluation on the additional benefit of new active substances on the adoption behaviour of physicians
Database (DOI: 10.13140/RG.2.2.18391.16809)
In order to investigate the research questions, the key element of the project was the double collection of all decisions according to AMNOG in the form of a database. For this purpose, all decisions concluded by the G-BA between January 2011 and December 2015 were recorded by student assistants. For each assessed active substance, the collected data points record data on the evidence submitted by the manufacturer, the G-BA's decision and its evaluation of the evidence, the evaluation of IQWIG, the price negotiations of the GKV-Spitzenverband (the leading association of SHI insurers in the German statutory health insurance system), and information on drug prices according to Lauer-Taxe. A total of 170 decisions on 137 active substances were recorded. This corresponds to 215 indications and 527 patient groups. For each active ingredient, usually several patient groups are recorded with potentially different indications for which the decision results differ. Furthermore, there is variation in the available evidence at this level. Figure 1 shows the link between the different levels:
Figure 1: Observation levels recorded in the AMNOG database
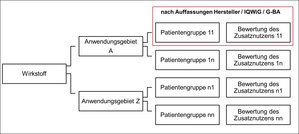
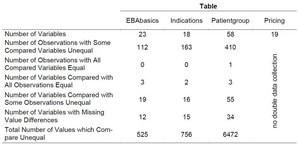
Table 1: Differences between two surveys of the AMNOG benefit assessment
On demand ,the database is available for research pruposes.
Results
For a more detailed overview, please refer to the Final Report to the DFG.
- K E, Blankart; T, Stargardt: The impact of drug quality ratings from health technology assessments on the adoption of new drugs by physicians in Germany. In: Health Economics, Jg. 2020 (2020). doi:https://doi.org/10.1002/hec.4108
- Fischer KE, Heisser T, Stargardt T. Health benefit assessment of pharmaceuticals: An international comparison of decisions from Germany, England, Scotland and Australia. Health Policy. 2016 Oct;120(10):1115–22.; Available at: http://www.sciencedirect.com/science/article/pii/S0168851016302044
- Lauenroth VD, Stargardt T. Pharmaceutical Pricing in Germany: How Is Value Determined within the Scope of AMNOG? Value in Health [online first: 18. Mai 2017]; Available at: http://www.sciencedirect.com/science/article/pii/S1098301517302061
- Fischer KE, Peters K, Stargardt T. Information on product quality, change agency and product adoption. ACAD MANAGE PROC. 2017 Jan 1;2017(1):13580.; Available at: http://proceedings.aom.org/content/2017/1/13580
- Fischer KE, Stargardt T (2014): Early Benefit Assessment of Pharmaceuticals in Germany Manufacturers’ Expectations versus the Federal Joint Committee’s Decisions. Med Decis Making. 34(8):1030–47.; Available at: http://journals.sagepub.com/doi/abs/10.1177/0272989X14546377
