The impact of regulations at the regional level using the example of biosimilars
Project description
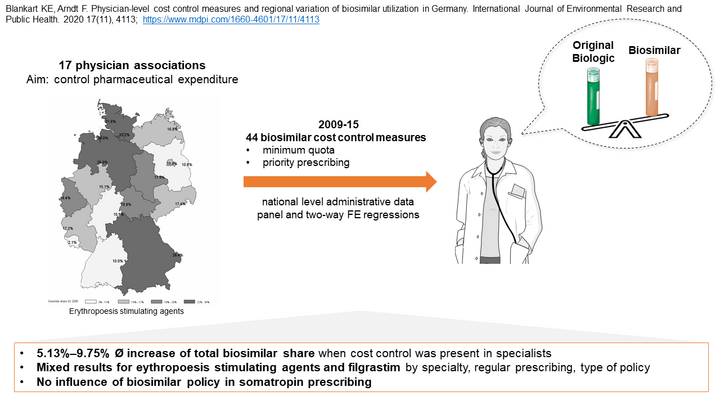
After more than ten years since the introduction of the first biosimilar in Germany, the regional prescription behaviour of resident and by health insurances registered physicians for this group of drugs varies considerably.This could be caused by diverging incentives for economic prescribing, such as minimum quantity requirements or the priority prescription of biosimilars at regional level. Within the framework of the project funding 'Versorgungsforschung 2016' of the 'Zentralinstitut für die Kassenärztliche Versorgung in Deutschland' (Zi) we investigate the influence of different control mechanisms on the prescribing behaviour of physicians.
Summary (Source: Final report to the Zi)
Introduction
Biosimilars are a less costly alternative to original biological drugs. Although only four percent of the insured receive biologics, they are responsible for 21 percent of drug expenditure. In addition, the proportion of biosimilar prescriptions in Germany varies greatly between regions. The aim of this analysis is to investigate the influence of the introduction of regulations (quotas, prioritized prescribing) by physician associations (PAs) on the prescribing behavior of physicians in Germany.
Methods
Our data consists of the drug agreements (Arzneimittelvereinbarungen) of the PAs and data sets of national outpatient services and prescription data for the period 2009-2015. We focus on the active substances somatropin, erythropoiesis stimulating agents (ESA) and filgrastim. Using suitable identification strategies (lagged-dependent variable approach / difference-in-difference analysis), the prescribing behavior of physicians in PAs with regulation is compared with that of physicians in PAs without regulation. Outcome variables are the proportion of biosimilar prescriptions and the number of prescriptions of biologics and biosimilars. We control for regional differences as well as practice and patient characteristics.
Results
On the one hand, we find positive effects for the frequently prescribed active substances ESA and filgrastim. On the other hand, physicians did not react to regulations for somatropin. Where significant effects were identified, the introduction of regulations increased the proportion of biosimilar prescriptions on average between 2.7 and 14 percentage points (p<0.01). Overall, a regionally heterogeneous picture emerges. For example, the number of prescriptions of original drugs decreases due to the introduction of the priority prescription in the PA Bremen, while opposite effects can be observed for the introduction of a quota in other PAs.
Discussion
The effect of regulation on the prescribing behavior for biosimilars is not uniform. There were no clear effects of regulation depending on the introduction of explicit quotas or priority prescription requirements. Due to unobservable factors such as price reactions by manufacturers and individual discount agreements of insurers, it is not possible to determine the causal effect of the measures conclusively yet.
References
K E, Blankart; F, Arndt: Physician-Level Cost Control Measures and Regional Variation of Biosimilar Utilization in Germany. In: International Journal of Environmental Research and Public Health, Jg. 2020 (2020) Nr. 17. doi:https://doi.org/10.3390/ijerph17114113
Birkner B, Blankart KE. The Effect of Biosimilar Prescription Targets for Erythropoiesis-Stimulating Agents on the Prescribing Behavior of Physicians in Germany. Value in Health. 2022 May 5; Available from: https://www.sciencedirect.com/science/article/pii/S1098301522001474

